Classification and Nomenclature of Organic Compounds
I. 1. What is Organic Chemistry and Why is Nomenclature Important?
Organic Chemistry Definition: Study of carbon-containing compounds, their structures, properties, reactions, and synthesis.
Importance: Foundational to life, medicine, materials, and various industries.
Scale: Millions of organic compounds exist due to carbon's unique bonding.
Carbon Bonding: Forms stable covalent bonds with itself and other elements (H, O, N, halogens, P). Can be single, double, or triple.
Examples of Organic Chemistry in Daily Life: Burning a match (cellulose combustion), food (carbohydrates), clothes (polymers), medicines.
Biological Significance: Carbohydrates, lipids, proteins, nucleic acids are organic molecules. Crucial for biochemistry and medicine (drug development).
Materials Science: Polymers (plastics, fibers, rubber) are organic. Properties depend on structure.
Industrial Applications: Agrochemicals, dyes, detergents, specialized chemicals, petrochemicals.
Nomenclature Importance: Enables clear communication about organic compound identity and structure.
1.1 The Importance of Systematic Nomenclature
Problem with Trivial Names: Ambiguous, inconsistent, don't convey structural information (e.g., formic acid, urea).
Systematic Nomenclature (IUPAC): Standardized, logical system based on structure. Universal language for chemists.
Key Aspects of Importance:
1.2 The Language of Chemistry: Communication and Standardization
Nomenclature as a Language: Facilitates clear communication and collaboration among chemists globally.
Transcends Boundaries: Enables international collaboration despite language differences.
Standardization: Consistent rules for deriving names. Allows deducing structure from name and vice versa.
Community and Shared Understanding: Common language for discussing structures, reactions, and properties.
Digital Realm: Essential for chemical databases, modeling software, and electronic notebooks.
1.3 Historical Context: Early Naming Systems vs. Modern IUPAC
Early Naming (Trivial Names): Based on source, properties, discoverer (e.g., acetic acid, citric acid, morphine).
Challenges of Trivial Names: Difficult to memorize, no structural information, inconsistencies, hindered progress.
Development of Structural Theory (Mid-19th Century): Emphasized the need for structure-based naming.
Geneva Nomenclature (1892): First major attempt at systematic naming. Introduced key principles (longest chain, suffixes).
Formation of IUPAC (1919): Goal to establish a unified international system.
IUPAC Nomenclature (1930 onwards): Regularly updated rules for naming diverse organic structures.
Shift to IUPAC: Significant advancement, providing a common language for chemists, aiding communication and knowledge dissemination.
1.4 Basic Concepts: Atoms, Bonds, Functional Groups
Carbon Atom: Central to organic chemistry. Tetravalent (forms 4 bonds).
Common Elements: H, O, N, S, P, halogens.
Chemical Bonds: Forces holding atoms together. Predominantly covalent (sharing electrons).
- Single Bond: Sharing 1 electron pair.
- Double Bond: Sharing 2 electron pairs.
- Triple Bond: Sharing 3 electron pairs.
Electronegativity: Ability of an atom to attract electrons. Leads to polar covalent bonds.
Formal Charge and Resonance: Important for representing electron structure.
Functional Group: Specific group of atoms responsible for characteristic reactions. Key to nomenclature.
Common Functional Groups (Examples):
- Alkanes: C-C single bonds (unreactive).
- Alkenes: C=C double bond (more reactive).
- Alkynes: C≡C triple bond (highly reactive).
- Alcohols: -OH group.
- Ethers: R-O-R' group.
- Aldehydes: C=O bonded to at least one H.
- Ketones: C=O bonded to two alkyl/aryl groups.
- Carboxylic Acids: -COOH group.
- Esters: -COOR group.
- Amines: Nitrogen bonded to alkyl/aryl groups.
- Amides: C=O bonded to nitrogen.
- Halides: Halogen bonded to carbon.
Importance of Functional Groups: Determine reactivity and are incorporated into IUPAC names.
2. Fundamental Principles of Organic Structure
3D Arrangement: Understanding the spatial arrangement of atoms is crucial.
2.1 Carbon's Unique Bonding Properties
Tetravalency: Carbon forms four covalent bonds. Due to sp3 hybridization (tetrahedral, 109.5°).
Hybridization: Can also undergo sp2 (planar, 120°, double bonds) and sp (linear, 180°, triple bonds) hybridization.
Catenation: Ability to form stable bonds with other carbon atoms (chains, branches, rings).
Bonding with Other Nonmetals: Forms strong bonds with H, O, N, S, P, halogens. Bond type and polarity influence properties.
2.2 Drawing and Interpreting Organic Structures (Lewis Structures, Line-Angle/Skeletal Formulas, Condensed Formulas)
Lewis Structures (Dot Structures): Show all valence electrons (bonding and non-bonding). Useful for visualizing electrons and connectivity. Cumbersome for large molecules.
Example: Methane, Water.
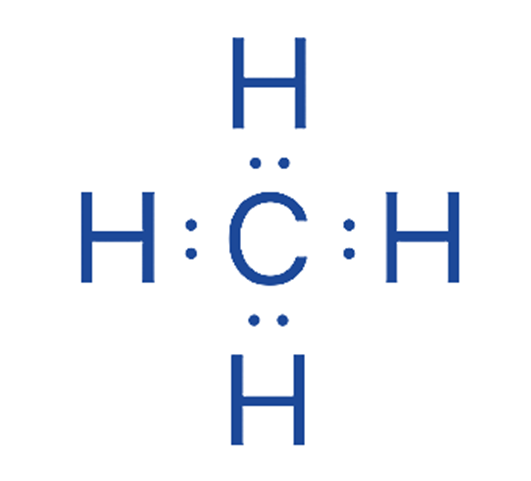
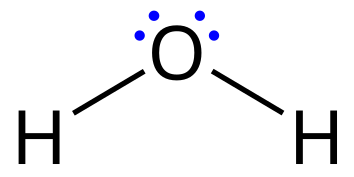
Line-Angle/Skeletal Formulas (Zig-Zag Structures): Simplified representation. Carbons at line ends/intersections, hydrogens implied. Heteroatoms shown. Emphasizes carbon skeleton. Quick to draw.
Example: Butane, Ethanol.

Condensed Formulas: Textual representation, grouping atoms. Branches in parentheses. Double/triple bonds sometimes shown. Saves space. Can be ambiguous.
Example: Butane (CH3CH2CH2CH3), Ethanol (CH3CH2OH).
2.3 Representing 3D Structures: Wedge and Dash Notation, Perspective Drawings
Wedge and Dash Notation: Shows 3D arrangement around an atom (typically tetrahedral carbon).
- Solid Lines: In plane.
- Solid Wedges: Out of plane (towards viewer).
- Dashed Wedges: Into plane (away from viewer).
Importance: Illustrating chirality and distinguishing stereoisomers.
Example: A carbon atom bonded to four different groups.

Perspective Drawings: Visualize 3D arrangement, focusing on conformation around a bond.
- Newman Projections: View along a C-C bond axis. Front carbon as a circle center, back carbon implied (bonds from circle edge). Shows conformational relationships (staggered/eclipsed).
- Sawhorse Projections: View along C-C bond at an angle. Both carbons shown. Clearer view of spatial relationships.
Usefulness: Analyzing conformational isomers and steric interactions.
2.4 Bond Types and Polarity
Bond Types (Covalent):
- Single Bonds (σ): One electron pair shared (head-on overlap). Free rotation.
- Double Bonds (σ and π): Two electron pairs shared (head-on and sideways overlap). Restricted rotation. Stronger and shorter.
- Triple Bonds (σ and 2π): Three electron pairs shared. Restricted rotation. Strongest and shortest.
Bond Polarity: Unequal electron sharing due to electronegativity differences.
- Nonpolar Covalent: Equal sharing (similar electronegativity, e.g., C-C, C-H).
- Polar Covalent: Unequal sharing (different electronegativity, e.g., C-O, C-N, C-X). Partial charges (δ+, δ-).
Molecular Polarity: Overall polarity depends on bond polarities and molecular geometry.
Importance: Predicting reactivity, explaining physical properties, understanding molecular interactions.
2.5 Isomers: Structural (Constitutional) Isomers, Stereoisomers
Isomers Definition: Same molecular formula, different arrangement of atoms.
Structural (Constitutional) Isomers: Different connectivity of atoms.
- Types: Different carbon skeletons, different positions of functional groups, different functional groups.
- Example: Butane isomers, propanol isomers, dimethyl ether/ethanol.
- Properties: Different physical and potentially chemical properties.
Stereoisomers: Same connectivity, different spatial arrangement.
- Enantiomers: Non-superimposable mirror images. Chiral molecules (at least one stereocenter - carbon with 4 different groups). Identical physical properties (except optical rotation). Different interactions with chiral molecules.
-
Diastereomers: Not mirror images. Different physical
and chemical properties. Arise with multiple stereocenters where not
all configurations are opposite.
- Cis-Trans Isomers (Geometric Isomers): Due to restricted rotation (double bonds, rings). Cis: higher priority substituents on the same side. Trans: on opposite sides.
Significance of Isomerism: Different properties and biological activities (e.g., drug enantiomers).
Classification of Organic Compounds
3. Hydrocarbons: The Foundation of Organic Chemistry
Key Definitions:
- Hydrocarbons: Organic compounds containing only carbon and hydrogen atoms.
- Saturated Hydrocarbons: Hydrocarbons with only single carbon-carbon bonds (C-C).
- Unsaturated Hydrocarbons: Hydrocarbons with at least one carbon-carbon double (C=C) or triple (C≡C) bond.
- Aliphatic Hydrocarbons: Linear or branched chain hydrocarbons and cyclic hydrocarbons (excluding aromatic).
- Aromatic Hydrocarbons: Hydrocarbons containing a benzene ring (a stable six-membered ring with alternating single and double bonds).
3.1 Alkanes: Nomenclature, Properties, and Reactions
Definition: Alkanes = saturated hydrocarbons with C-C single bonds.
Example: Methane (CH₄), ethane (C₂H₆).
Nomenclature:
- Mnemonics: "Longest Chain, Lowest Numbers, Alphabetical Order."
-
Key Rules:
- Identify the longest continuous carbon chain.
- Number carbons to give substituents the lowest numbers.
- Name substituents as alkyl groups (e.g., methyl, ethyl).
- List substituents alphabetically.
- Use di-, tri-, tetra- for multiple identical substituents.
- Quick Example: Name CH₃-CH(CH₃)-CH₂-CH₃ (Answer: 2-methylbutane)
Properties:
-
Table: Properties of the First Five Alkanes
Alkane Molecular Formula Boiling Point (°C) State at Room Temp Methane CH₄ -162 Gas Ethane C₂H₆ -89 Gas Propane C₃H₈ -42 Gas Butane C₄H₁₀ -0.5 Gas Pentane C₅H₁₂ 36 Liquid -
General Trends:
- Increasing boiling point and melting point with increasing molecular weight (due to stronger London Dispersion Forces).
- Insoluble in water (nonpolar).
- Less dense than water.
 Reactions:
Reactions:
-
Key Reactions:
- Combustion: React with oxygen to produce carbon dioxide and water (releases energy). Example: $CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O$
- Halogenation (Radical Substitution): Reaction with halogens (Cl₂, Br₂) in the presence of UV light or heat, replacing a hydrogen atom with a halogen. Example: $CH_4 + Cl_2 \rightarrow CH_3Cl + HCl$
3.2 Cycloalkanes: Nomenclature and Ring Systems
Definition: Cycloalkanes = saturated hydrocarbons with carbon atoms joined in a ring.
Example: Cyclopropane (C₃H₆), cyclohexane (C₆H₁₂).
Nomenclature:
-
Key Rules:
- Add the prefix "cyclo-" to the parent alkane name.
- If there's only one substituent, no number is needed.
- If there are multiple substituents, number the ring to give the substituents the lowest possible numbers.
- List substituents alphabetically.
- Quick Example: Name the structure with a cyclopentane ring and a methyl group attached. (Answer: methylcyclopentane)
Ring Systems:
- Small Rings (Cyclopropane, Cyclobutane): Significant ring strain due to bond angle deviation from the ideal 109.5°. More reactive.
- Common Rings (Cyclopentane, Cyclohexane): Adopt non-planar conformations (e.g., chair conformation for cyclohexane) to minimize strain. More stable.

3.3 Alkenes: Nomenclature, Cis-Trans Isomerism, and Properties
Definition: Alkenes = unsaturated hydrocarbons containing at least one carbon-carbon double bond (C=C).
Example: Ethene (C₂H₄), propene (C₃H₆).
Nomenclature:
-
Key Rules:
- Identify the longest continuous carbon chain containing the double bond.
- Number the carbons so the double bond gets the lowest possible number.
- Change the "-ane" ending of the parent alkane to "-ene".
- Indicate the position of the double bond with a number before the parent name.
- Name substituents as before.
- Quick Example: Name $CH_3-CH=CH-CH_3$ (Answer: but-2-ene)
Cis-Trans Isomerism (Geometric Isomerism):
- Requirement: Restricted rotation around the double bond and two different groups on each carbon of the double bond.
- Cis Isomer: Substituents on the same side of the double bond.
- Trans Isomer: Substituents on opposite sides of the double bond.
!(cis-but-2-ene and trans-but-2-ene)[https://www.vedantu.com/question-sets/2b92d53e-d0bd-4cf4-afc7-5122c035d3435308516844318259558.png]
- Quick Example: Identify if $CH_3-CH=CH-CH_2CH_3$ can have cis-trans isomers (Yes).
Properties:
- Physical Properties: Similar to alkanes with similar carbon numbers, but slightly lower boiling points due to less efficient packing.
- Reactivity: More reactive than alkanes due to the presence of the pi bond.
-
Key Reactions:
-
Addition Reactions: The pi bond breaks, and new
atoms or groups are added to the carbon atoms.
- Hydrogenation: Addition of H₂ (requires a catalyst, e.g., Pt, Pd, Ni). Example: $CH_2=CH_2 + H_2 \rightarrow CH_3CH_3$
- Halogenation: Addition of Cl₂ or Br₂. Example: $CH_2=CH_2 + Br_2 \rightarrow CH_2BrCH_2Br$
- Hydrohalogenation: Addition of HX (HCl, HBr, HI). Follows Markovnikov's rule (hydrogen adds to the carbon with more hydrogens). Example: $CH_3CH=CH_2 + HBr \rightarrow CH_3CHBrCH_3$(major product)
- Hydration: Addition of water (requires acid catalyst). Follows Markovnikov's rule. Example: $CH_3CH=CH_2 + H_2O \rightarrow CH_3CH(OH)CH_3$ (major product)
-
Addition Reactions: The pi bond breaks, and new
atoms or groups are added to the carbon atoms.
- Mnemonics (Markovnikov's Rule): "The rich get richer" (hydrogen adds to the carbon with more hydrogens).
3.4 Alkynes: Nomenclature and Properties
Definition: Alkynes = unsaturated hydrocarbons containing at least one carbon-carbon triple bond (C≡C).
Example: Ethyne (acetylene, C₂H₂), propyne (CH₃C≡CH).
Nomenclature:
-
Key Rules:
- Similar to alkene nomenclature, but change the "-ane" ending to "-yne".
- Number the carbons to give the triple bond the lowest possible number.
- Quick Example: Name $CH_3-C\equiv C-CH_3$ (Answer: but-2-yne)
Properties:
-
Physical Properties: Similar to alkanes and alkenes of comparable size, but slightly higher boiling points than corresponding alkenes due to stronger intermolecular forces.
-
Reactivity: Highly reactive due to the presence of two pi bonds.
-
Key Reactions:
-
Addition Reactions: Similar to alkenes, but can
add two moles of reagent due to two pi bonds.
- Hydrogenation: Can be controlled to form alkenes (using Lindlar's catalyst) or alkanes (using excess H₂ and standard catalysts).
- Halogenation: Can add one or two moles of halogen.
- Hydrohalogenation: Follows Markovnikov's rule for the first addition.
- Hydration: Addition of water in the presence of HgSO₄ and H₂SO₄ forms an enol, which tautomerizes to a ketone. Example: $HC\equiv CH + H_2O \rightarrow [CH_2=CHOH] \rightarrow CH_3CHO$ (acetaldehyde)
-
Addition Reactions: Similar to alkenes, but can
add two moles of reagent due to two pi bonds.
-
Table: Comparing Reactivity (Qualitative)
Hydrocarbon Reactivity Alkanes Low Alkenes Medium Alkynes High
3.5 Aromatic Hydrocarbons: Benzene, Substituted Benzenes, and Polycyclic Aromatic Hydrocarbons
Definition: Aromatic Hydrocarbons = hydrocarbons containing a benzene ring, a six-membered ring with alternating single and double bonds (exhibiting resonance).
Example: Benzene (C₆H₆), toluene (methylbenzene, C₇H₈).
Benzene (C₆H₆):
- Structure: Cyclic, planar molecule with six carbon atoms and alternating single and double bonds. Exhibits resonance, meaning the electrons are delocalized, making all C-C bonds equivalent in length and strength.
- Stability: Highly stable due to resonance. Undergoes substitution reactions rather than addition reactions like alkenes.
Substituted Benzenes:
- Nomenclature (Monosubstituted): Name the substituent followed by "benzene". Example: chlorobenzene, nitrobenzene.
- Nomenclature (Disubstituted): Use prefixes ortho- (1,2), meta- (1,3), and para- (1,4) to indicate the relative positions of the two substituents. Example: o-dichlorobenzene, m-nitrotoluene, p-bromophenol.
- Nomenclature (Polysubstituted): Number the ring to give substituents the lowest possible numbers.
-
Electrophilic Aromatic Substitution: The
characteristic reaction of benzene. An electrophile (electron-seeking
species) replaces a hydrogen atom on the benzene ring.
-
Key Reactions:
- Halogenation: Reaction with Cl₂ or Br₂ in the presence of a Lewis acid catalyst (e.g., FeCl₃, FeBr₃).
- Nitration: Reaction with concentrated nitric acid and sulfuric acid.
- Sulfonation: Reaction with fuming sulfuric acid (SO₃ in H₂SO₄).
- Friedel-Crafts Alkylation: Reaction with an alkyl halide in the presence of a Lewis acid catalyst (e.g., AlCl₃).
- Friedel-Crafts Acylation: Reaction with an acyl halide or anhydride in the presence of a Lewis acid catalyst.
-
Key Reactions:
-
Directing Effects of Substituents: Substituents
already on the benzene ring influence where the next substituent will
attach.
- Activating and ortho, para-directing groups: Donate electron density to the ring (e.g., -OH, -NH₂, -R).
- Deactivating and meta-directing groups: Withdraw electron density from the ring (e.g., -NO₂, -COOH, -CHO).
- Halogens: Deactivating but ortho, para-directing.
Polycyclic Aromatic Hydrocarbons (PAHs):
- Definition: Aromatic hydrocarbons containing two or more fused benzene rings.
- Examples: Naphthalene, anthracene, phenanthrene.
-
Significance: Some PAHs are carcinogenic
(cancer-causing).
4. Functional Groups: The Heart of Reactivity
4.1 Definition of a Functional Group
- Specific group of atoms within a molecule that is responsible for the characteristic chemical reactions of that molecule.
- The reactive center of an organic molecule.
- Dictates the molecule's chemical behavior.
4.2 Classification by Functional Group
4.2.1 Halides (Alkyl and Aryl Halides)
- Definition: A halogen atom (F, Cl, Br, I) bonded to an alkyl (alkyl halide) or aryl (aryl halide) group.
- Example: CH₃Cl (chloromethane), C₆H₅Br (bromobenzene).
- Key Characteristic/Reactivity: Undergo nucleophilic substitution and elimination reactions. Aryl halides are less reactive towards SN reactions.
4.2.2 Alcohols, Phenols, and Ethers
4.2.3 Aldehydes and Ketones
4.2.4 Carboxylic Acids and Their Derivatives (Esters, Amides, Acid Anhydrides, Acyl Halides)
4.2.5 Amines