Lewis Structures
Lewis structures are mainly useful in three ways: they help you understand how electrons are arranged around atoms, they make it easier to visualize molecular geometry, and they remind you where lone pairs are located. In Organic Chemistry 1, you'll see that both the shape of molecules and the position of lone pairs strongly influence reactivity. That's why reviewing Lewis structures is a valuable exercise.
What are Lewis structures?
Lewis structures are diagrams that show how electrons are distributed between atoms in a molecule. For example, here are the Lewis dot structures of beryllium dichloride, borane, methane, ammonia, water, and hydrofluoric acid.
The Full Lewis
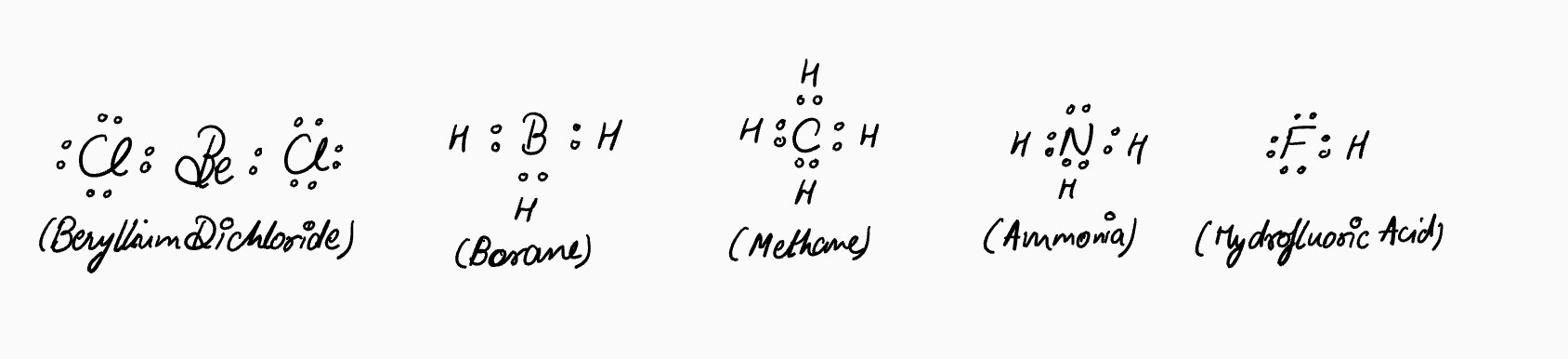
The benefit of drawing the full Lewis structure is that it lets you see all the electrons and check if each atom follows the octet rule. You can clearly distinguish bonding pairs (shared between atoms) from non-bonding pairs, or lone pairs.
Think of the full Lewis structure like training wheels on a bike—it’s especially helpful when you’re first learning about atoms, electrons, and molecules, and want reassurance that the octet rule generally holds true. Keep in mind, though, that exceptions exist, such as with electron-deficient atoms like beryllium and boron.
That said, full Lewis structures can quickly become tedious to draw. Once you’re comfortable with the basics, it’s easier to use line bonds, as shown below. This style also makes it simpler to illustrate geometry, since the drawing looks less cluttered.
The "Half-Lewis"
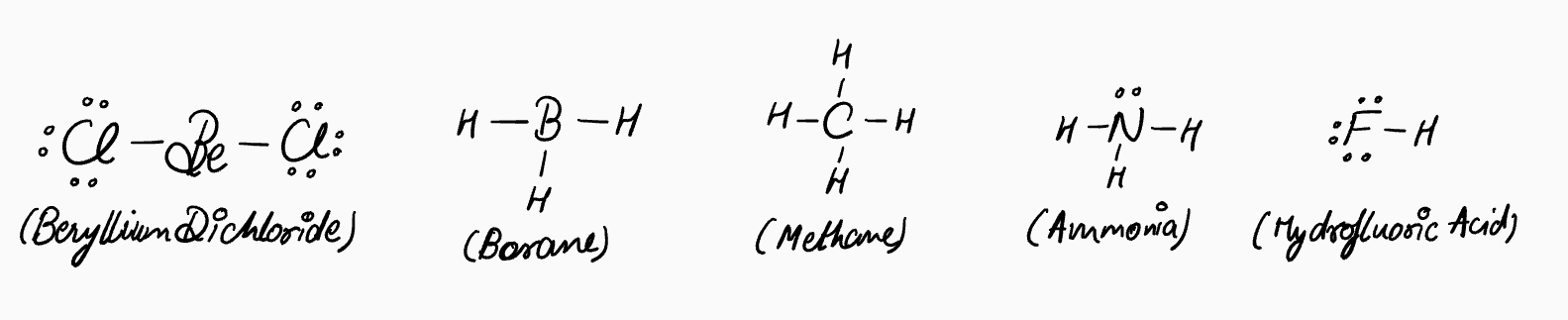
Here’s the key point: electron pairs repel each other. This applies to both bonding pairs and lone pairs. Molecules adopt shapes that maximize the distance between these pairs. That’s why methane is tetrahedral (angles of about 109°) instead of square planar (90° angles), and why water is bent rather than linear. This principle is called VSEPR (valence shell electron pair repulsion).
Drawing molecular geometry with full Lewis structures gets messy fast. That’s why we switch to half-Lewis structures—using line bonds, moving them around, and leaving in only the electron pairs when needed.
Using Lewis Structures for Molecular Geometry
Line structures are especially effective for showing molecular geometry:
, BH3 (trigonal planar), CH4 (tetrahedral), NH3 (trigonal pyramidal), H2O (bent), and HF (linear)..png)
Molecules with Multiple Bonds
The same idea applies to molecules with double or triple bonds:
, Carbon dioxide (linear), Ethyne (linear).png)
At this stage, many chemists take an even shorter route: leaving out lone pairs altogether. This shorthand style is the most common way to represent molecules. Here are a few examples:
Shorthand (or "Lazy") Structures
Examples of this shorthand approach:
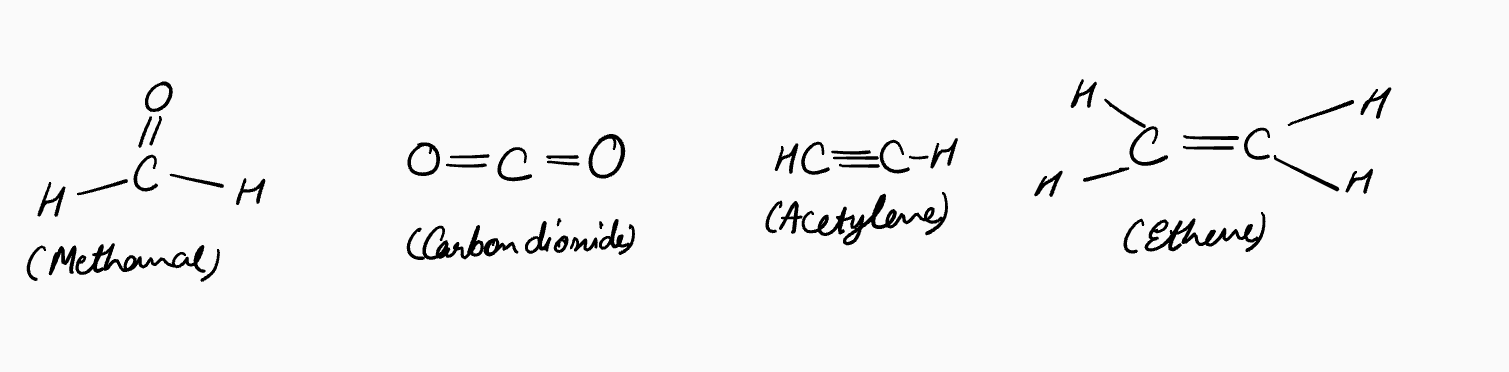
Even if lone pairs aren’t drawn, you should assume they’re still present. Think of these diagrams like stick figures in comics—just because details like hands or faces aren’t shown doesn’t mean they’re missing; it’s simply faster to draw. This matters because in organic chemistry, lone pairs don’t just sit idle. They often act as nucleophiles, actively participating in many reactions. So while the shorthand skips drawing them, you must always keep them in mind.