How Does One Even Know Methane (CH₄) Is Tetrahedral?
What Do The Valence Electrons Of Carbon Tell Us About The Bonding In CH₄?
If the ground-state orbital configuration of carbon is 2s²2p², how does this information help us determine the arrangement of atoms in a simple molecule like methane (CH₄)? It turns out that experimental evidence shows methane is tetrahedral, with four equal bond angles of 109.5°, four equal bond lengths, and no net dipole moment. This leads to two key questions: First, how do we know for certain that CH₄ is tetrahedral? And second, how do we reconcile carbon's 2s²2p² electronic configuration with the fact that methane has four identical C-H bonds?
Summary: The Tetrahedral Structure of Methane
- The electronic configuration of elemental carbon is 2s²2p². If carbon simply used these atomic orbitals to bond with four hydrogen atoms, we would expect three hydrogens to bond with the 2p orbitals (pₓ, pᵧ, p₂) and one hydrogen to bond with the 2s orbital.
- This arrangement would lead to unequal C-H bond lengths and different bond angles, resulting in a net dipole moment (since the individual C-H bond dipoles would not cancel out).
- However, careful measurements confirm that methane (CH₄) has no dipole moment and that all four C-H bonds are of equal length and strength.
- This discrepancy is resolved by the concept of hybridization. The bonding orbitals in methane are not "pure" 2s or 2p orbitals, but are "hybrid" orbitals formed from their combination.
- Specifically, one 2s orbital and three 2p orbitals "hybridize" to form four equivalent sp³ hybrid orbitals.
- We now know methane is tetrahedral because this arrangement maximizes the distance between the repulsive electron pairs in the bonds (109.5° angle), as predicted by VSEPR theory, making it more stable than a flat, square planar geometry (90° angle).
- Fun fact: In the 1870s, Jacobus van't Hoff deduced that bonding around carbon must be tetrahedral, as a square planar structure could not explain the observed phenomenon of optical isomerism.
1. The Electronic Configuration Of Carbon's Valence Electrons Is 2s²2p²
As a refresher on atomic orbitals, the valence electron configuration of a carbon atom is 2s²2p², meaning it has two electrons in the 2s orbital and one electron in each of two different 2p orbitals, with one 2p orbital remaining empty.
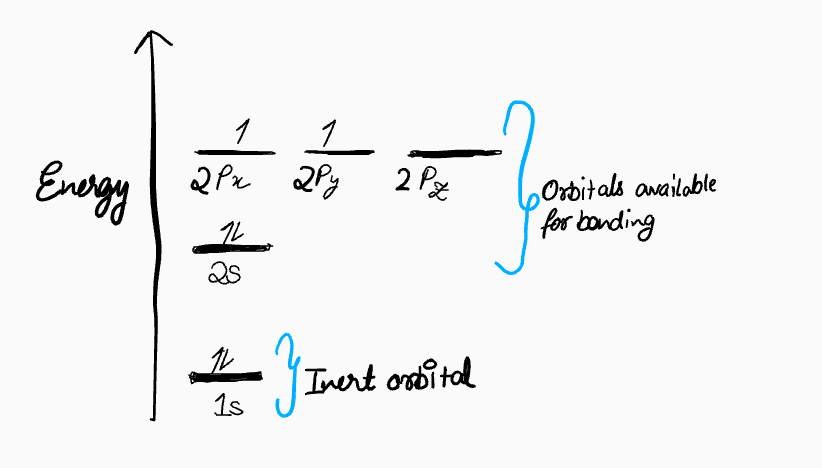
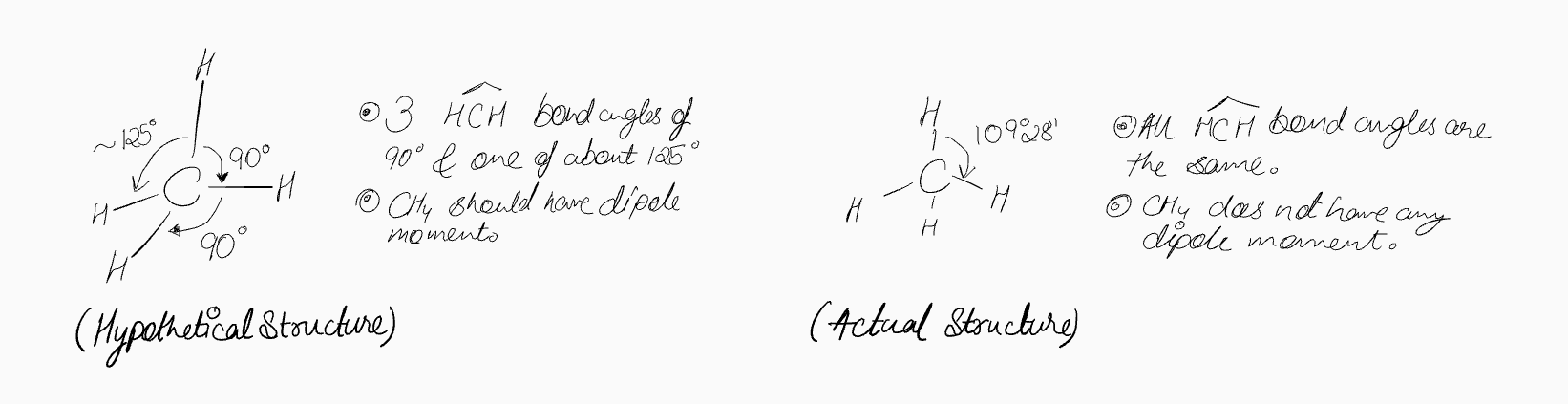
2. A Flawed Hypothesis: Using Atomic Orbitals Directly
If we were to construct a model of methane using carbon's ground-state atomic orbitals, we might make a reasonable but incorrect guess. Since the three p-orbitals (pₓ, pᵧ, p₂) are oriented at 90° to each other along the x, y, and z axes, one might expect three C-H bonds to form along these axes with 90° bond angles. The fourth C-H bond, formed with the spherical 2s orbital, would repel the other bonds, perhaps settling at an angle like 135°.
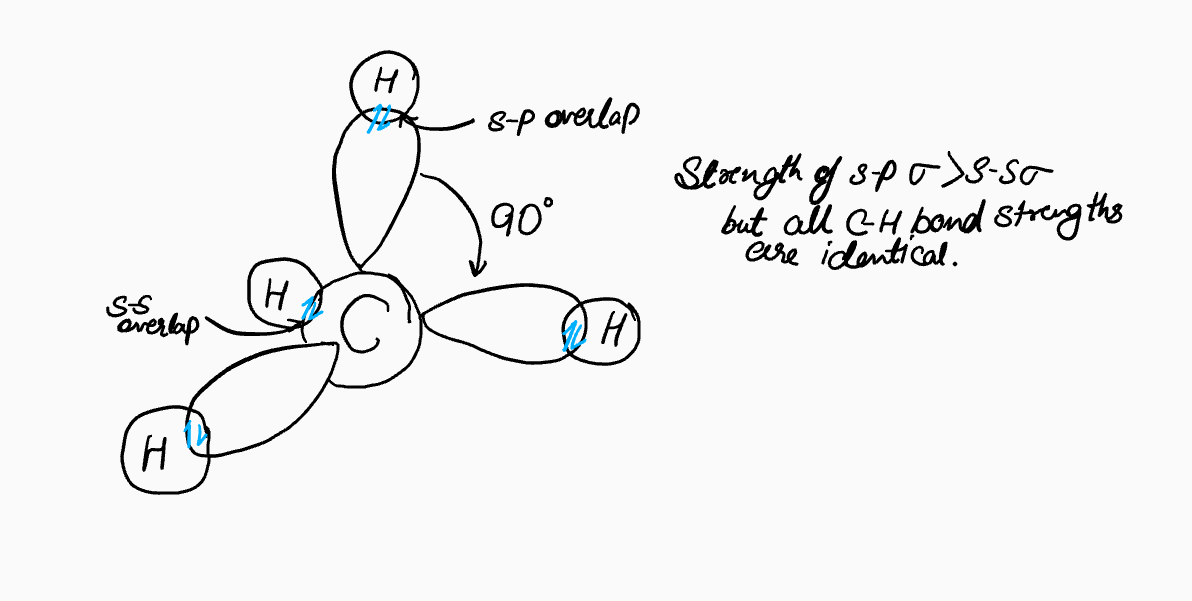
This proposed structure is incorrect for several reasons. Each C-H bond has a small dipole due to the electronegativity difference between carbon (2.5) and hydrogen (2.2). In this hypothetical structure, the vector sum of these dipoles would not cancel out, resulting in a net dipole moment for the molecule. However, experiments show that the dipole moment of methane is zero. This indicates that the molecule must be perfectly symmetrical, with all bond lengths and angles being identical.
3. Disproving the Square Planar Structure
If all bonds must be identical, another possibility is a flat, "square planar" structure, with 90° bond angles. This was, in fact, the prevailing theory until the late 1800s. However, we now know this is also wrong.
The key evidence came from the study of optical isomerism. If a carbon atom is bonded to four different groups (e.g., in bromochlorofluoromethane, CHBrClF), it can exist as two different isomers that are non-superimposable mirror images of each other, much like a left and right hand. These isomers are called enantiomers.
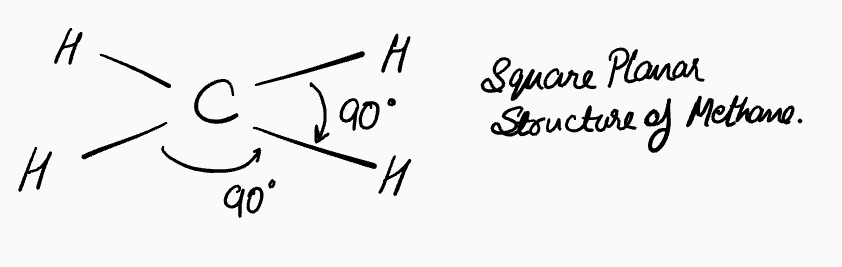
This phenomenon of "chirality" is only possible if the four groups are arranged tetrahedrally around the central carbon. A square planar arrangement would be flat and its mirror image would be superimposable, meaning only one compound could exist. Since two isomers of compounds like CHBrClF have been isolated, the square planar model must be incorrect.
In 1874, Jacobus Henricus van't Hoff was among the first to propose the three-dimensional, tetrahedral nature of carbon, correctly explaining the existence of optical isomers.
4. Hybrid Orbitals: The Modern Explanation
We now rationalize the tetrahedral geometry of methane using VSEPR (Valence Shell Electron Pair Repulsion) theory, which states that electron pairs in bonds will arrange themselves to be as far apart as possible. A tetrahedron with 109.5° angles achieves this separation perfectly.
But how do we reconcile this geometry with the 90° angles of p-orbitals and the spherical shape of the s-orbital? The answer lies in hybrid orbitals. The conventional treatment is that carbon's one 2s orbital and three 2p orbitals combine to form four new, identical orbitals called sp³ hybrid orbitals. These hybrid orbitals are directed towards the corners of a tetrahedron, perfectly explaining the observed bond angles and equal bond lengths in methane.