Ionic and Covalent Bonding
The concept of chemical bonding is rooted in Coulomb's law, which describes the attraction and repulsion between charges. This fundamental force governs how atoms interact and is responsible for the properties of molecules.
Two key principles are at play:
- Atoms form relationships called chemical bonds by sharing pairs of electrons.
- Atoms possess a property called electronegativity, which measures their ability to attract electrons.
This leads to an important consequence: when a bond forms between atoms with different electronegativities, the electron pair is not shared equally. Instead, it is polarized, or pulled, toward the atom with the higher electronegativity. This means the more electronegative atom gains a partial negative charge (higher electron density), while the less electronegative atom develops a partial positive charge (lower electron density).
The Polarity Spectrum
The greater the difference in electronegativity between two atoms, the more polarized the bond. Sometimes, the "sharing" of electrons is so unequal it barely qualifies as sharing at all. Imagine two brothers, ages 10 and 8, who are given $20 "to share." Without parental oversight, the older, stronger brother will likely control most, if not all, of the money. Though they are nominally sharing, the power dynamic dictates the outcome.
It's the same with atoms. At one extreme, you have a bond between a highly electronegative element like fluorine (F) and a weakly electronegative one like lithium (Li). In lithium fluoride (LiF), the bond is so polarized that the electron is essentially transferred from lithium to fluorine. This creates charged atoms, or ions (Li⁺ and F⁻), held together by electrostatic attraction. This is known as an ionic bond.
At the other extreme, when two identical atoms like fluorine bond to each other (F-F), there is no difference in electronegativity. The electrons are shared perfectly equally. This type of bond, a full and equal partnership, is called a covalent bond.
Most chemical bonds exist on a spectrum between these two extremes. The difference in electronegativity (
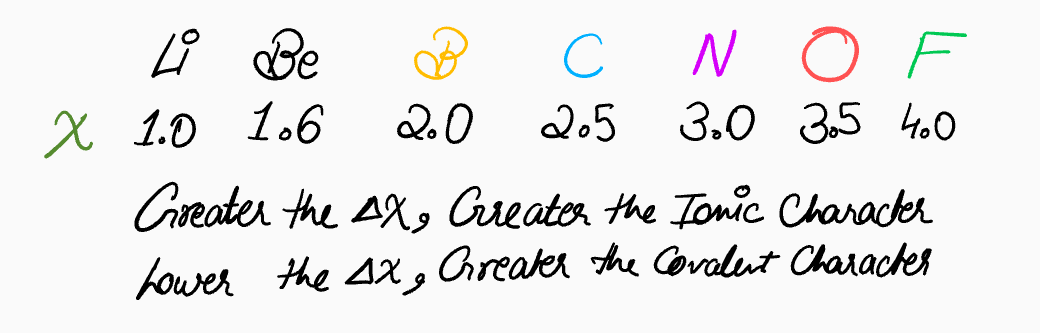
It's crucial to avoid thinking of bonds as strictly ionic OR covalent. Instead, it's more accurate to consider their "flavor" or "character." Most bonds are polar covalent, possessing a mix of both ionic and covalent characteristics.
Implications in Organic Chemistry
This principle of a bonding spectrum is fundamental to understanding the richness of chemistry. Its implications are vast, but here are a few key examples:
- Boiling/Melting Points: Generally, the more polarized a molecule is, the stronger the intermolecular attractions, leading to higher boiling and melting points. Water (H₂O), a highly polar molecule, has an exceptionally high boiling point of 100°C for its size.
- Solubility: The slogan "like dissolves like" applies here. Polar molecules tend to dissolve well in polar solvents (like water), while nonpolar molecules dissolve in nonpolar solvents.
- Acidity: The electronegativity of the atom bonded to hydrogen is a major factor in determining a molecule's acidity.
- Reactivity: This is especially important in organic chemistry. Regions of high electron density (partial negative charges) will be attracted to regions of low electron density (partial positive charges). This guides how molecules interact and react.
Learning to recognize these dipoles is a key skill for predicting reactivity. It forms the basis for understanding electrophilicity (electron-deficiency) and nucleophilicity (electron-richness).
(Advanced) References and Further Reading
- Principles of electronegativity Part I. General nature. R. T. Sanderson, Journal of Chemical Education 1988 65 (2), 112. DOI: 10.1021/ed065p112
- Principles of electronegativity Part II. Applications. R. T. Sanderson, Journal of Chemical Education 1988 65 (3), 227. DOI: 10.1021/ed065p227
These two papers by R.T. Sanderson provide a clear and concise discussion of electronegativity, its historical measurement, potential improvements, and the importance of partial charges in understanding chemical reactions.